Makrite Impacting the N95 Mask Shortage
At the beginning of 2020 there was a N95 mask shortage, the N95 mask cost about $1 each, and medical professionals disposed of them after each patient visit. With just over three months remaining in 2020, masks have quintupled in price, and top government agencies have recommendations for “extended” or “optimized” use.
Nearly three-quarters of more than 20,000 nurses surveyed reuse respirators, according to the Washington Post. Some nurses claim to use a NIOSH N95 mask for months. Hospitals attempt to disinfect reused masks when necessary with UV light or hydrogen peroxide. Companies like Technology Solutions Group (TSG) provide N95 mask disinfection and tracking solutions to prolong the masks’ effectiveness.
The FDA reported that many hospitals made PPE orders based on previous usage and not future need. In part, hospitals used this practice based on the assumption that the U.S. stockpile could help during emergencies. In 2009, however, the H1N1 flu epidemic severely depleted the NIOSH N95 mask stockpile. The government failed to replenish the stockpile, according to the Washington Post.
CDC and FDA Recommendations
The CDC and FDA made “extended” and “optimized” recommendations instead of the White House creating a coherent national plan. According to NPR, the White House contends that manufacturing 160 million masks per month will quench the need. Public health officials claim the U.S. will be hundreds of millions of masks short of the 3.5 billion masks needed this year.
N95 Demand
With the COVID-19 vaccine, a distant hope, and more than 200,000 deaths in the United States, the global shortage of N95s forced NIOSH, the CDC, and the FDA to push the limits on N95s and other PPE.
Historic wildfires on the West Coast amplified the need for N95s, according to a recent Forbes column. Cloth masks, while effective against COVID-19, are of little use filtering wildfire smoke. N95s effectively filter the microscopic particles of wildfire smoke, but the supply doesn’t match the demand.
The White House opted for a big-firm focus with masks, leaving out small- and mid-sized manufacturers. Despite their expertise and size, 3M and Honeywell can’t meet the current need for N95s by themselves.
FDA-Approved Options
To meet the need, the CDC and FDA approved Chinese-made KN95s along with other measures. U.S. hospitals that tried the KN95s found the fit insufficient and discontinued use.
NIOSH Certified Respirators and the N95 Mask Shortage
Makrite has an entire catalog of NIOSH certified respirators that includes the model 9500-N95, which is FDA approved as a surgical respirator. This Makrite N95 mask presents a high-quality replacement for 3M and Honeywell masks and many hospitals have been using this N95 Mask prior to COOVID. As the most extensive, private-brand manufacturer of face masks and disposable respirators, Makrite boasts more than 40 NIOSH approved safety products.
Meltblown Shortage
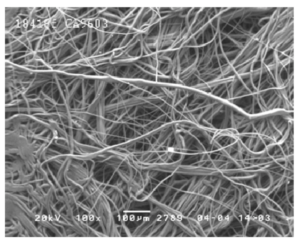
Engineers designed the N95 mask as single-use. N95s filter at least 95% of airborne particles while forming a seal around the nose and mouth. Polypropylene fibers, roughly 1/50th the size of a human hair, make up the meltblown textile that creates an electrostatic web to attract particles.
The Associated Press reported that a lack of meltblown textile is holding up the N95 production process. Some U.S. manufacturers continue to export meltblown textile despite the shortage.
Manufacturers refuse to ramp production of meltblown textile because the White House refuses to guarantee purchase once the need subsides. After the H1N1 virus outbreak, some companies experienced bankruptcy and laid off employees because of a glut of supply without demand.
Defense Production Act
According to the Washington Post, the AMA, AHA, American Nurses Association, AFL-CIO requested the Defense Production Act’s broader use. The DPA uses presidential power over funding for the production and distribution of critical supplies in a crisis.
While the U.S. did force the ramp-up of ventilator production early in the pandemic, it never involved itself with PPE production. As a result, companies must balance current needs with future needs and their organization’s financial health.
U.S. Manufactures Face Shields
Some U.S. manufacturers took the initiative early in the pandemic by making face shields, which require much less production than N95 masks. NPR reported companies left with a glut of face shields amidst an N95 shortage.
SPH Medical and Makrite Filling the Gaps
The quality and prolific Makrite N95 mask manufacturing coupled with SPH Medical’s reliable supply chain expertise means N95 relief is on the way. The combination of Makrite and SPH Medical equals more model 9500-N95 delivery to hospitals, frontline caregivers, and others in need. SPH Medical is also working with TSG to explore validated mask disinfection for its customers.

