Description
Sienna Kits for Covid-19 Antigen Testing
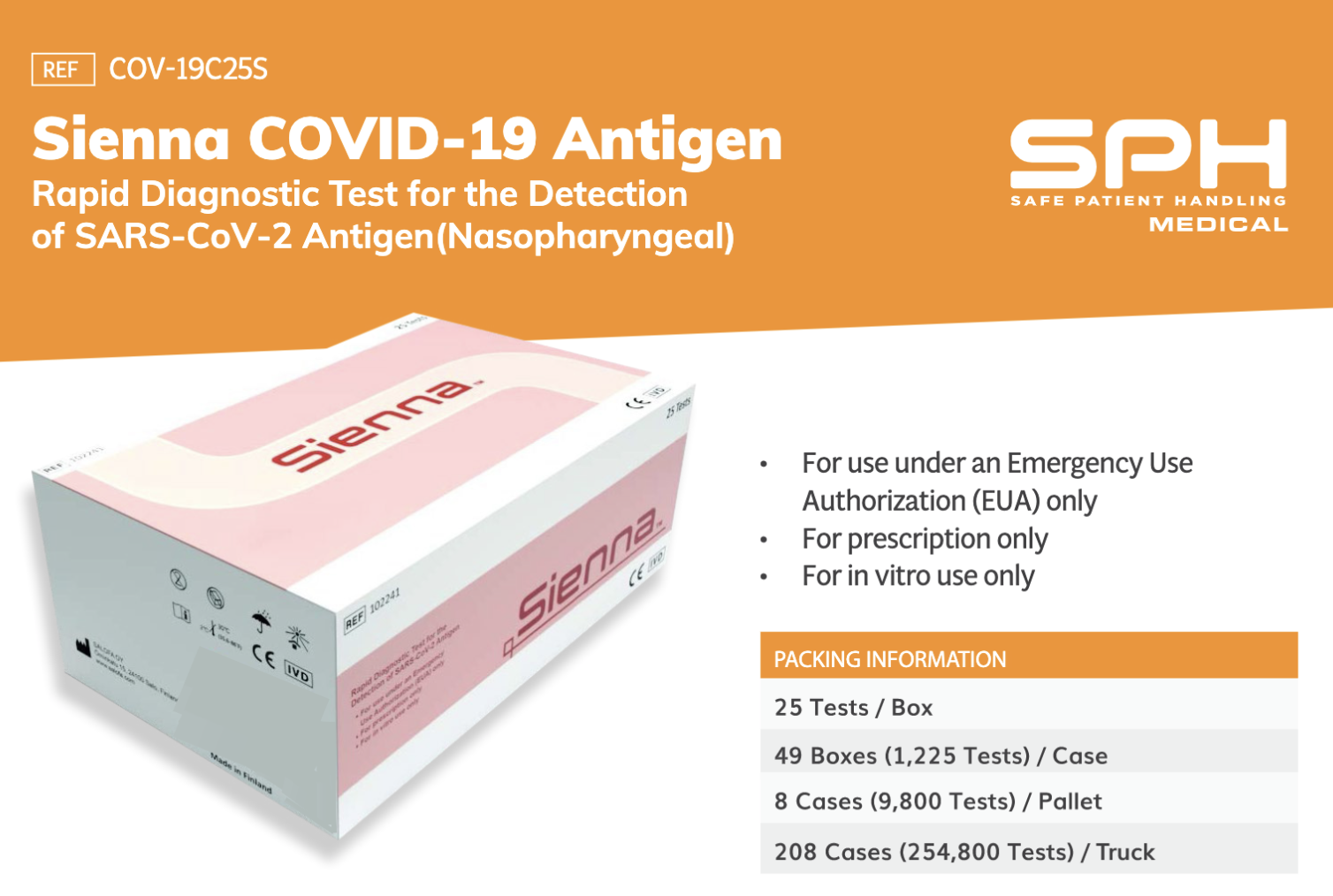
SPH Medical offers The Sienna COVID-19 Antigen Rapid Test Cassette for in vitro use only and can detect the existence of SARS-CoV-2 Antigen. This is a prescription-only diagnostic test, which utilizes a nasopharyngeal swab to determine if an individual has Covid-19.
Authorized healthcare providers, may recommend this Point of Care testing within the first 6 days of the onset of symptoms. Each box of the Sienna Rapid Covid-19 Antigen Test has 25 individual rapid chromatographic immunoassay tests. Through the use of direct nasopharyngeal swabs (NP), specimens of the nucleocapsid protein antigen from SARS-CoV-2 are detected.
The authorization of these tests is based upon having a CLIA FDA cleared/approved, and authorized by FDA. This must be under an EUA for use only by authorized laboratories and performed in a patient care setting.
Sienna COVID-19 Antigen Rapid Test Kits Contain
The processing of the Sienna Rapid Test Cassette is approved to take place in a laboratory. This testing is limited under the Clinical Laboratory Amendments of 1988. It is important to note that this test does not differentiate between SARS-CoV and SARS-CoV-2. All US laboratories are required to report their findings to relevant health authorities. Each kit includes everything necessary to perform antigen testing.
Inside each SPH Medical product kit you will find:
- Extraction Buers
- Sterile NP Swabs
- Quick User Guide
- Negative Control
- Instructions for Use
- Individually Pouched Tests Cassettes
- Workstation
- Positive Control Swab
Prompt Convenience
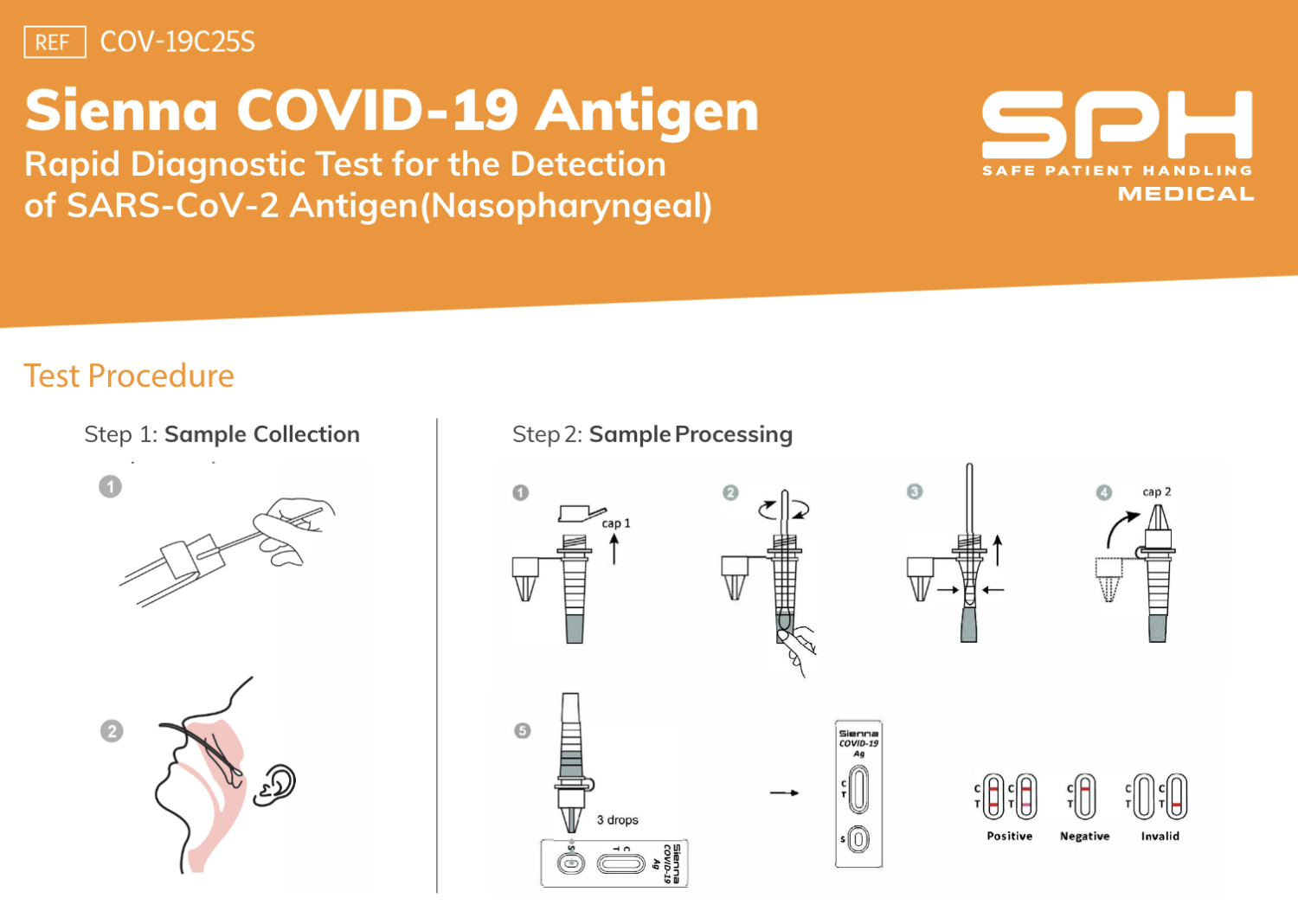
This particular Covid-19 Antigen Test is both rapid and easy to use. As authorized by the FDA, Sienna Kits provide test results within 10 minutes. Those performing the test can conveniently do so without additional equipment or required training. The results here will show through qualitative detection whether the nucleocapsid protein antigen is found. It is possible to simultaneously test multiple individuals with extra buer vials and Nasopharyngeal swabs.
Testing Preparation
There are required steps in order to prepare for the use of Sienna Kits for testing. The temperature of the cassette does make a difference. The healthcare professionals should ensure that the cassette, specimen, and extraction buffer are at room temperature. This means remaining at 15°C – 30°C (59°F – 86°F) prior to any testing efforts. Following the Quick User Guide and the Instructions for Use is recommended.
Evidence-Based Testing
This Sienna Covid-19 Antigen Test underwent performance evaluations before becoming available for clinical use. A prospective, non-interventional study was performed in a Point of Care setting to verify results. This process involved the use of 133 Nasopharyngeal swab samples, which were collected from individuals presenting symptoms representative of the Covid-19 infection. A US FDA authorized RT-PCR functioned as a reference to confirm results for antigen testing.
To accommodate wide-scale testing activities, the Sienna Rapid Test Kits can be purchased in bulk. A case of this product contains 49 individual boxes, which equates to 1,225 tests. It is possible to also purchase pallets and truck-size loads of this product.
Contact SPH Medical to receive a quote for the purchase of this antigen testing product based upon your facility’s needs.