Description
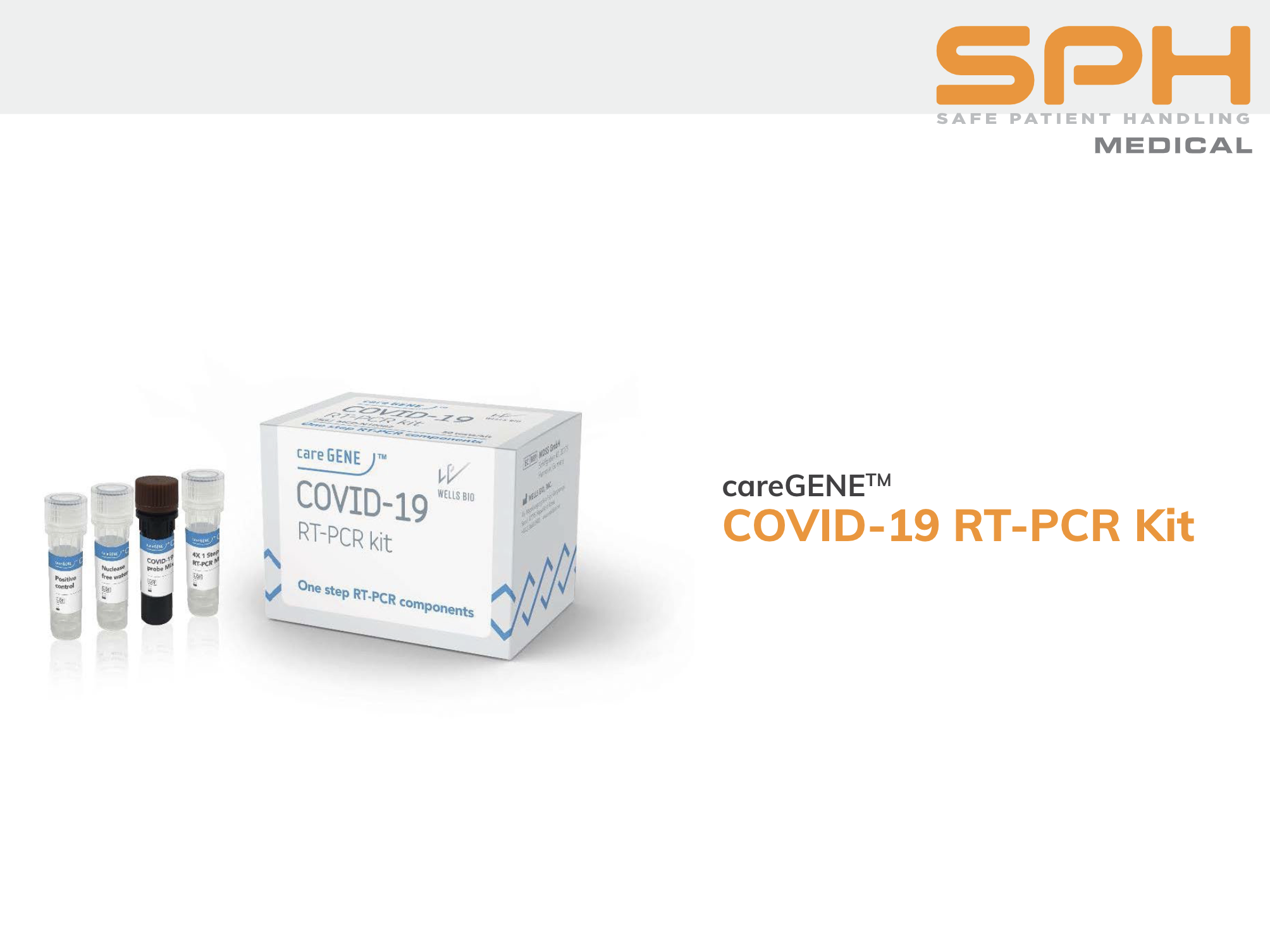
CareGENE™ COVID-19 RT-PCR Kit
The CareGENE™ COVID-19 PCR test kit has received emergency use authorization in the United States and South Korea to diagnose COVID-19 infection. A variety of upper respiratory samples, including nasopharyngeal, oropharyngeal, and anterior nasal swabs, may be used with the kits.
AccessBio’s one-step reverse transcription PCR technology can detect two different genetic targets (RNA-dependent RNA polymerase/RdRp and the N gene) in the same well. This simultaneous one-tube/two-target design ensures reliability, calibrated to an analytical sensitivity of 10 copies/uL, without compromising turnaround time at 83 minutes.
Each COVID-19 RT-PCR kit was designed with durability in mind and is good for 12 months at storage below -20°C.
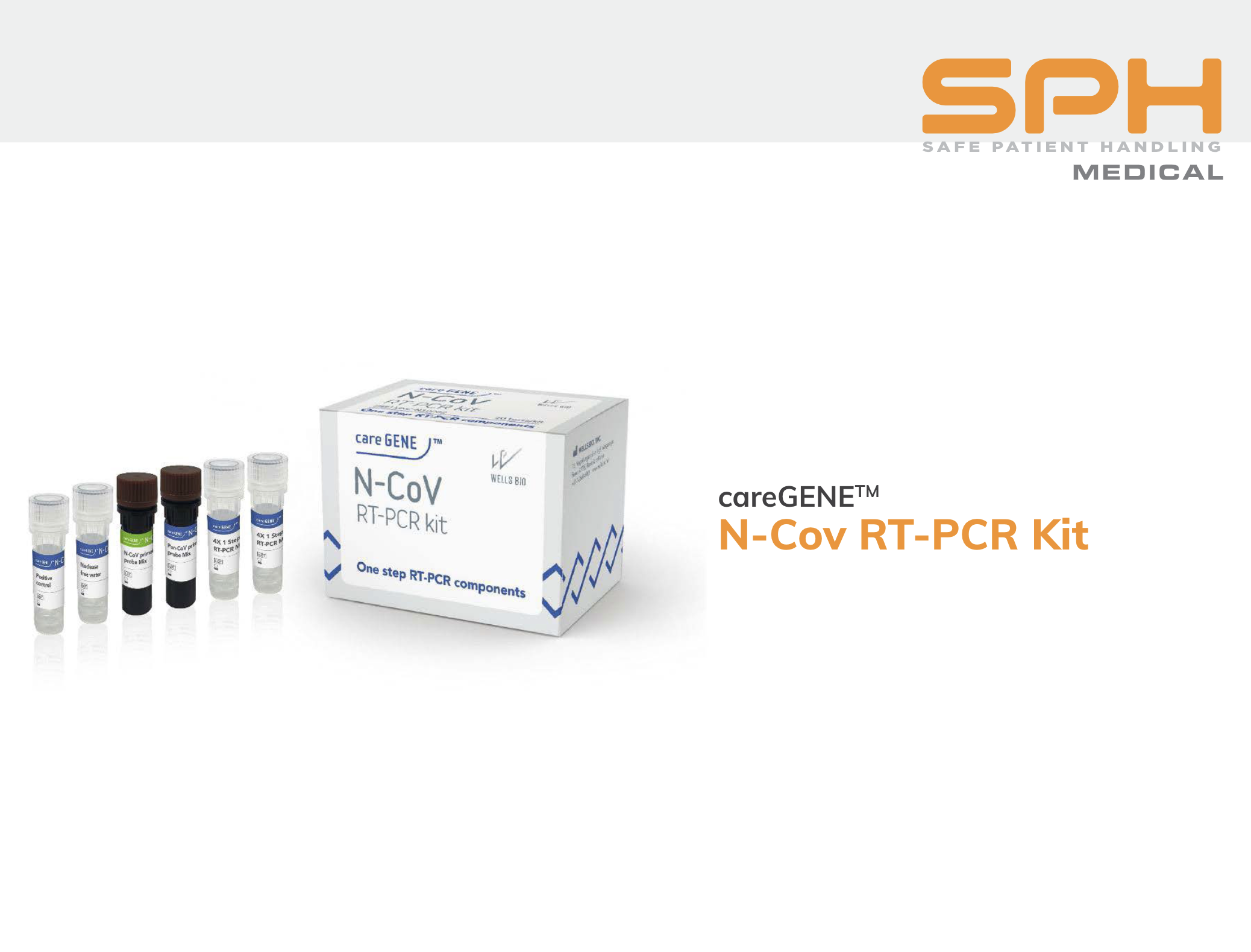
CareGENE™ N-COV RT-PCR Kit
NOTE: The N-COV RT-PCR Kit does not have EUA in the United States.
The CareGENE™ N-COV RT-PCR test kit is designed to diagnose beta-CoV and other novel coronavirus infections in real time. As with the COVID-19 PCR test kit, the N-COV kit can use a variety of respiratory samples including nasopharyngeal, oropharyngeal, or anterior nasal swabs.
The test kits use a two-tube, two-target design, calibrated to a sensitivity of 5 copies/uL, to ensure comprehensive detection and reliable results. Each test will detect both RNA-dependent RNA polymerase (RdRp) and the viruses’ E gene in the same reaction well, with internal controls present to simplify interpretation. Turnaround time from swab to result takes 83 minutes on average.
Each test kit was designed for durability and has a shelf life of 6 months at storage below -20°C.
EUA and Testing Indications
Though each test kit detects SARS-CoV2 at different targets, they share similar indications across the countries in which they have received emergency use authorization. The kits are intended for use in patients suspected of having COVID-19 regardless of symptomatic status. However, they can only be used to detect current, not prior, infection.
Positive results indicate the confirmed presence of SARS-CoV2, but do not rule out co-infections. Improper sample collection may yield false positive or negative results. Negative results, though more reliable with an RT-PCR test compared to an antigen test, do not preclude infection with SARS-CoV2.
Testing must be carried out by qualified personnel in certified laboratory settings, specifically those which meet CLIA requirements to perform high-complexity tests. Results must be interpreted in the context of all other information available to the provider.
How to Order
SPH Medical supplies each of the two PCR test kit options in bulk to providers. Only test kits approved for use in your region or country will be available to order. Click on “Get A Quote” to obtain pricing information.